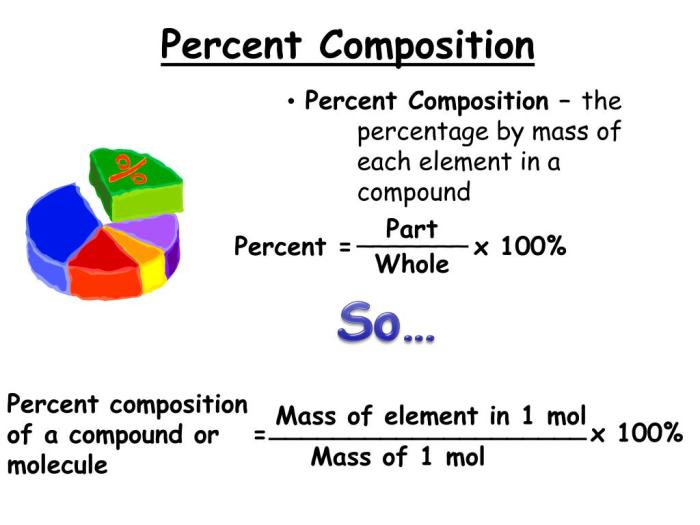
Percent composition empirical and molecular formulas color by number – Embark on an enlightening journey into the realm of chemistry with percent composition, empirical and molecular formulas, brought to life through the vibrant hues of color. This captivating exploration unveils the intricate connections between these fundamental concepts, revealing their significance in deciphering the composition of matter.
Delving into the depths of percent composition, we unravel the art of expressing the elemental makeup of compounds as percentages. Empirical formulas, the simplest whole-number ratios of atoms, provide a glimpse into the composition of compounds without revealing their molecular structure.
Molecular formulas, on the other hand, unveil the exact number of atoms of each element present in a molecule, offering a more comprehensive understanding of its structure.
Percent Composition: Percent Composition Empirical And Molecular Formulas Color By Number
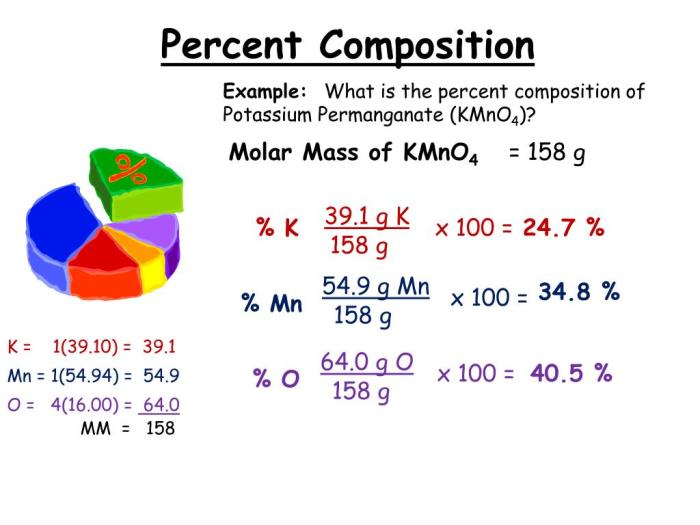
Percent composition refers to the relative amount of each element in a compound expressed as a percentage of the total mass. It provides a quantitative description of the elemental makeup of a substance.
To calculate percent composition, the mass of each element in the compound is divided by the total mass of the compound and multiplied by 100. For instance, if a compound has 12 g of carbon, 2 g of hydrogen, and 16 g of oxygen, the percent composition would be:
- Percent composition of carbon = (12 g / 30 g) x 100 = 40%
- Percent composition of hydrogen = (2 g / 30 g) x 100 = 6.67%
- Percent composition of oxygen = (16 g / 30 g) x 100 = 53.33%
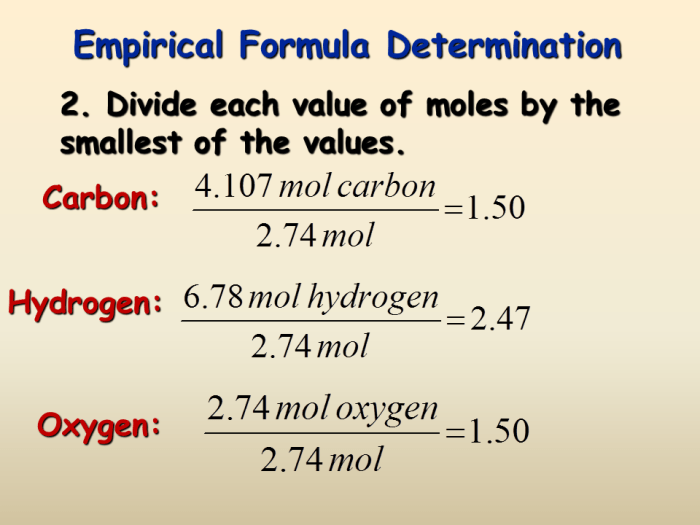
Empirical Formulas
An empirical formula represents the simplest whole-number ratio of elements in a compound. It does not necessarily indicate the actual molecular structure or the number of atoms of each element in a molecule.
To determine the empirical formula from percent composition, the following steps are taken:
- Convert the percent composition to grams by assuming a 100 g sample.
- Convert the grams of each element to moles by dividing by its respective atomic mass.
- Find the simplest whole-number ratio of moles by dividing each mole value by the smallest mole value.
- Multiply the mole ratios by the appropriate subscripts to obtain the empirical formula.
For example, a compound with 40% carbon, 6.67% hydrogen, and 53.33% oxygen has an empirical formula of CH 2O.
Empirical formulas differ from molecular formulas, which represent the actual number of atoms of each element in a molecule. Molecular formulas can be determined by considering the empirical formula and the molar mass of the compound.
Molecular Formulas
A molecular formula represents the exact number of atoms of each element in a molecule of a compound. To determine the molecular formula, the molar mass of the compound must be known.
The molar mass is the mass of one mole of a substance, which is equal to the sum of the atomic masses of all the atoms in the molecular formula. By comparing the molar mass to the empirical formula, the molecular formula can be deduced.
For example, if a compound has an empirical formula of CH 2O and a molar mass of 60 g/mol, the molecular formula is C 2H 4O 2because 2 x (12 + 2 + 16) = 60.
Color by Number
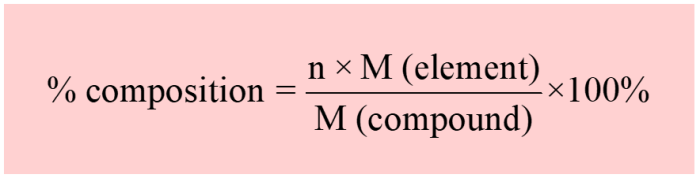
| Percent Composition | Empirical Formula | Molecular Formula |
|---|---|---|
| 40% C, 6.67% H, 53.33% O | CH2O | C2H4O2 |
| 54.55% C, 9.09% H, 36.36% O | C3H6O | C3H6O |
| 60% C, 4.00% H, 36.00% O | CH2O | C6H12O6 |
Legend:
- Red: Empirical formula and molecular formula are different.
- Green: Empirical formula and molecular formula are the same.
- Blue: Empirical formula and molecular formula have the same ratio but different subscripts.
The color-coded table provides a quick and easy way to interpret data and understand the relationships between percent composition, empirical formulas, and molecular formulas.
Question Bank
What is the significance of percent composition?
Percent composition provides a standardized method for expressing the elemental makeup of compounds, allowing for easy comparison and analysis of their composition.
How do empirical formulas differ from molecular formulas?
Empirical formulas represent the simplest whole-number ratios of atoms in a compound, while molecular formulas reveal the exact number of atoms of each element present in a molecule.
What role does molar mass play in determining molecular formulas?
Molar mass, the mass of one mole of a substance, serves as a bridge between percent composition and molecular formulas, enabling us to calculate the molecular formula of a compound based on its percent composition.